Quality leap for fluorenone flow battery
(sustainabilityenvironment.com) – Just a little sugar and the battery of flow marks a new record. It happened in the laboratories of the Pacific Northwest National Laboratory (PNNL), in the United States, where a research group has created a special fluorenone flow battery that has maintained its ability to store and release energy for more than a year of continuous charge and discharge. The results achieved were described in a study just published in the journal Joule. The article details the first use in the cellular architecture of a simple sugar called β-cyclodextrin, a derivative of starch now used as a food additive. But to understand its use we must take a few steps back.
What are flow batteries?
Flow batteries are an electrochemical storage technology that releases electrical energy through reversible oxidation and reduction of working fluids. The concept was born in the 70s, but only with the new century and the growing interest in the world of energy storage, technology has taken effect.
Unlike traditional rechargeable batteries, flow batteries have two external supply tanks containing the anolyte and the catholite, respectively. That is the electrolyte fluids in which the electroactive substances are dissolved. To provide electricity, the discharge phase, the liquids are pumped into the central electrochemical cell, where the oxide-reduction reaction takes place. Charging is done by simply replacing the liquid electrolyte. This architecture makes the storage capacity dependent on the volume of the tank (the larger it is, the greater the stored energy) and the concentration of electrolytes. Instead, the power is exclusively a function of the membrane surface of the cell.
Flow battery, advantages and disadvantages
But with unparalleled advantages in terms of scalability and design flexibility, as well as the independence between the accumulated energy and the power delivered, this technology faces several obstacles. In general, flow batteries have large cells with voluminous components for high performance, resulting in low volumetric power density, footprint and high capital costs. Not only. Although there are many projects in place and some commercial installations, those that have reached the market still rely on vanadium, an expensive and difficult to obtain mineral. Why? Because it is the one capable of offering the best performance.
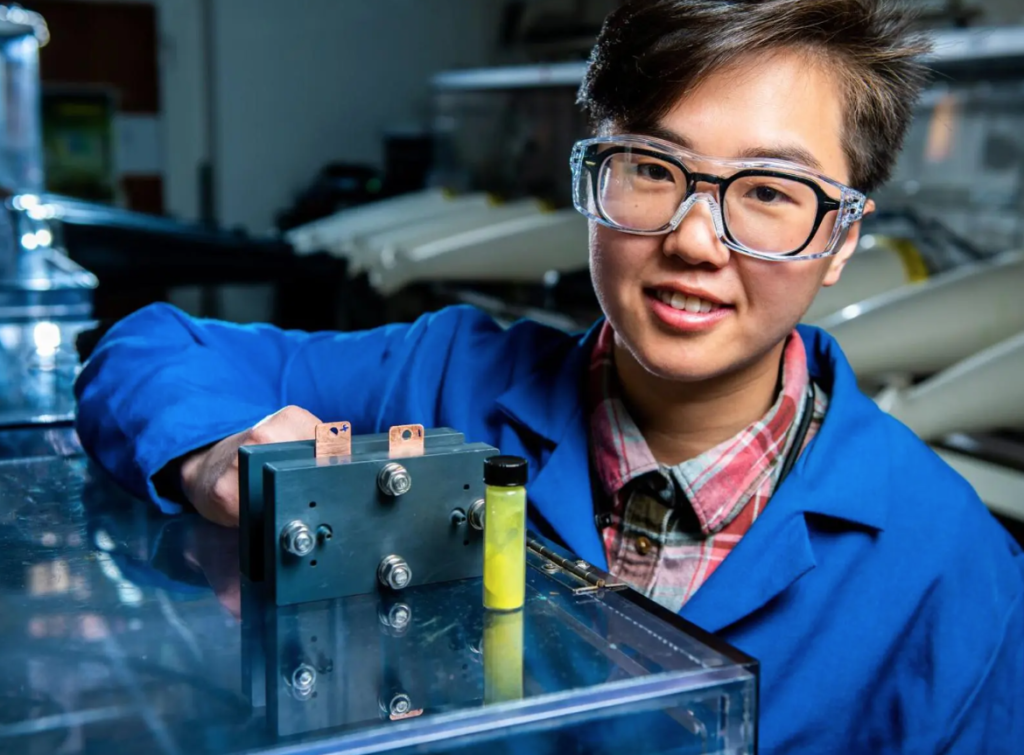
A pinch of sugar for fluorenone flow batteries
The NL team wanted an alternative to vanadium that was equally effective, but easy to synthesize and stable. The choice fell on fluorenone an organic compound used, among other things, to produce antimalarial drugs and candles. But this molecule was not water soluble enough nor did it show redox reversibility in aqueous solutions; So first the PNNL scientists had to create a series of complex chemical steps to transform fluorenone into a reversible, water-soluble redox compound.
However, to achieve a record-breaking fluorenone flow battery, performance had to be increased. The team added β-cyclodextrin, a compound that can accelerate the oxidation reaction and improve the overall kinetics of the battery.
In a series of experiments, scientists have optimized the ratio of chemicals in the system to reach 60% more peak power. So, they ran their unit over and over again for over a year, interrupting the experiment only by breaking a plastic tube. During all that time, the fluorenone flow battery showed a minimal capacity loss.